The XHCORR sequence
This sequence allows to correlate the signals of the
 and of the
and of the
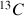 bound to
each other[1].
bound to
each other[1].
The spectrum 11 (XHCORR), shows the correlations for the dihydrofuran (Fig. 31) between bound carbons and protons.
The chemical shifts of the protons of this molecules are in the table 2, those of the carbon are in the table 3.
1H | Shift (ppm) |
|---|---|
A | 8.15 |
B | 7.4 |
C | 7.5 |
D | 7.7 |
13C | Shift (ppm) |
|---|---|
a | 156.2 |
b | 111.6 |
c | 127.0 |
d | 122.6 |
e | 120.6 |
f | 124.2 |
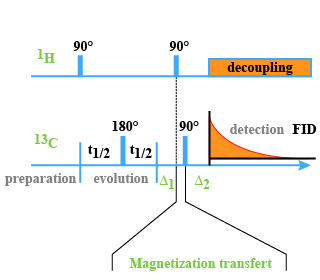
Within the XHCORR pulse sequence (Fig. 25), transverse magnetization is caused by a
 impulsion which is evolving during the
impulsion which is evolving during the
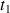 period. The
period. The
 impulsion
impulsion
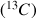 , located in the middle of this period refocuses the heteronuclear couplings.
, located in the middle of this period refocuses the heteronuclear couplings.
The optimization of the
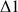 and
and
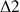 delays allows the selection of the long range heteronuclear couplings, this means that instead of seeing the correlation between
delays allows the selection of the long range heteronuclear couplings, this means that instead of seeing the correlation between
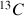 and protons directly bound
and protons directly bound
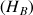 , we favour the appearance of the correlations spots between
, we favour the appearance of the correlations spots between
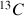 and non bound protons
and non bound protons
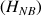 .
.
For example, for a coupling constant J=10 Hz then
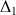 =50ms and
=50ms and
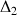 =33ms.
=33ms.